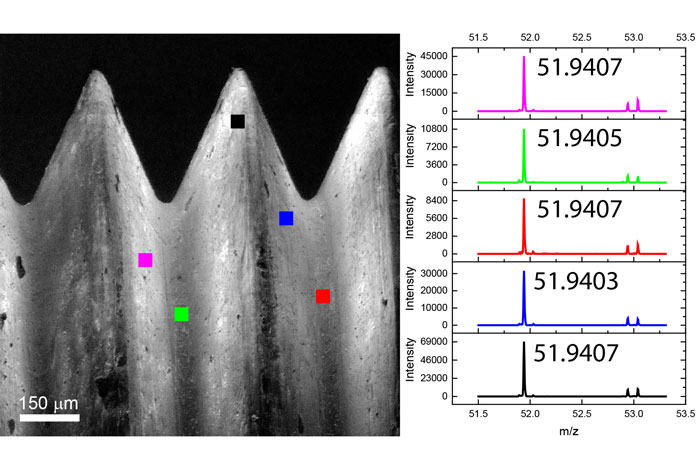
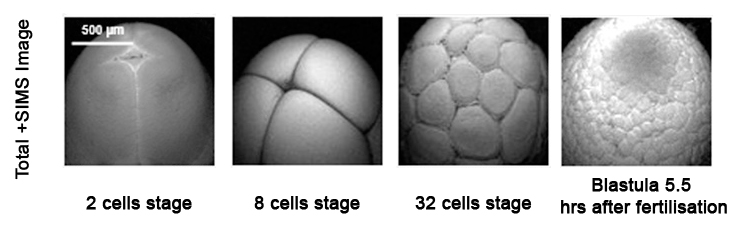
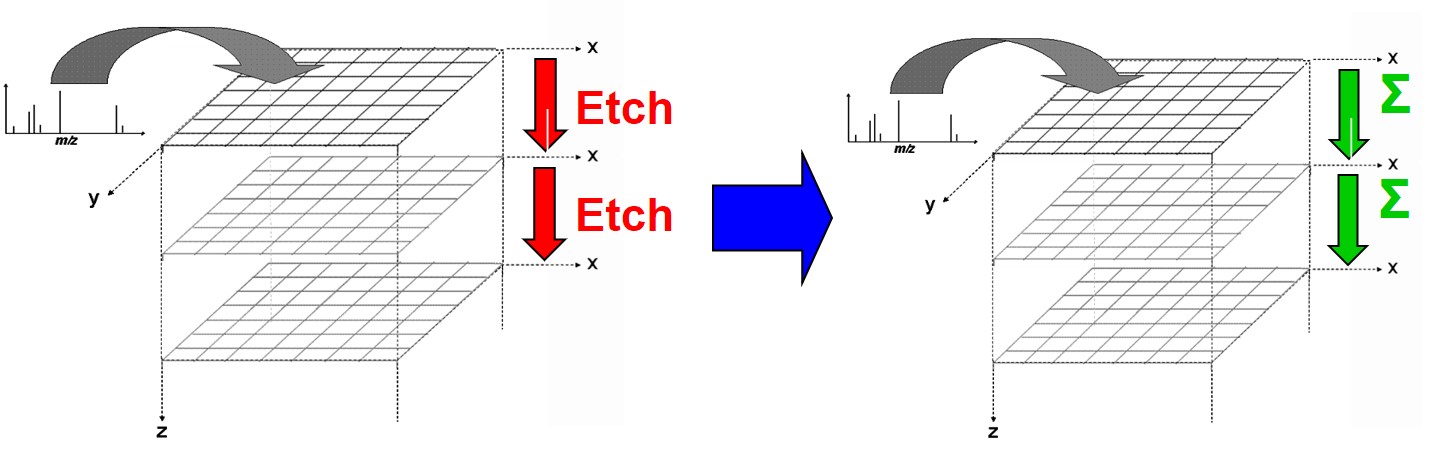
Ion Beams and their Applications
Ion beams come in many shapes and sizes, with multiple source options and applications. A minefield of options awaits if you are unfamiliar with them. This application note will shed some light on Ionoptika’s range of ion beams to help you choose the right one for your application.
Contents
- Sputter vs Analytical Ion Beams
- C60 Beams
- Gas Cluster Ion Beams
- Liquid Metal Ion Beams
- Plasma Ion Beams
- Conclusions
Sputter vs Analytical Ion Beams
We split our range of ion beams into two groups based on their applications or purpose – sputter beams and analytical beams.
Sputter Beams
While all ion beams will sputter a surface, we make this distinction based on the area and speed with which this occurs. Sputter beams have three characteristic features: high current, large spot size, and wide field of view. They deliver a large dose of ions over a wide area as quickly as possible to optimise etch rates.
Sputter beams remove material before analysis, either for cleaning purposes or for depth profiling through the sample. Techniques employing sputter beams include SIMS, XPS, SEM, TEM, and Auger.
Analytical Beams
Rather than being used to facilitate analysis using a different technique, analytical beams perform the analysis themselves. They also have three characteristic features; wide energy range, small spot size, and variable current control. These features give the user excellent control over the beam characteristics, enabling them to optimise their experiment.
Analytical beams are primarily used for secondary ion mass spectrometry (SIMS) and work well in traditional focused ion beam (FIB) applications such as secondary electron imaging and FIB milling.
C60 Beams
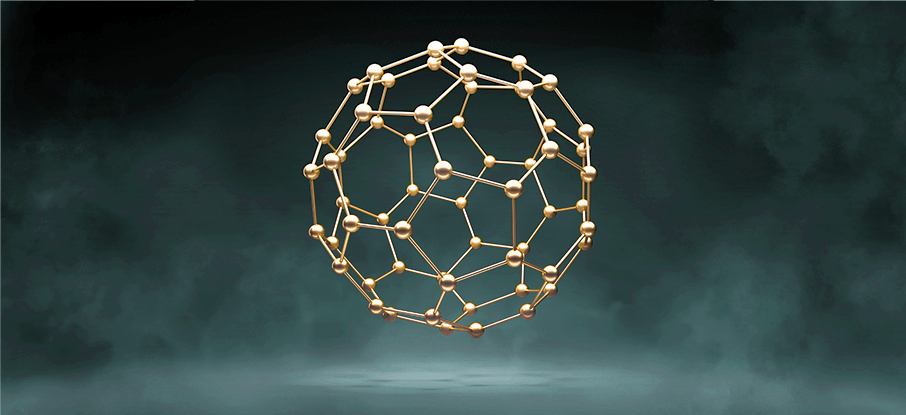
Carbon-60, or just C60, is a fullerene molecule consisting of sixty carbon atoms formed into a hollow sphere, with a shape very similar to a soccer ball. The first commercial C60 ion beam was produced in 2002 by Ionoptika in collaboration with the University of Manchester, and since then, we have sold more than 150 units worldwide.
Compared to monatomic ion beams, C60 beams result in a much “gentler” sputtering action, reducing molecular fragmentation and damage to sub-surface layers. When employed as an analytical beam, this gentle sputtering action significantly increases sensitivity to intact molecular ions.
As the C60 molecule is larger (~ 7 Å) than the lattice constant for most materials, it also does not channel through the lattice the way monatomic ions do, reducing preferential sputtering. C60 beams exhibit incredibly uniform sputter rates across a wide range of materials, including challenging poly-crystalline materials.
The properties of C60 make it suitable for both sputtering and analysis. Ionoptika offers three C60 ion beam systems: a broad-beam sputtering system, the C60-20S, and two analytical beams, the C60-20 and C60-40.
See our application note all about C60 beams for more information.
Gas Cluster Ion Beams
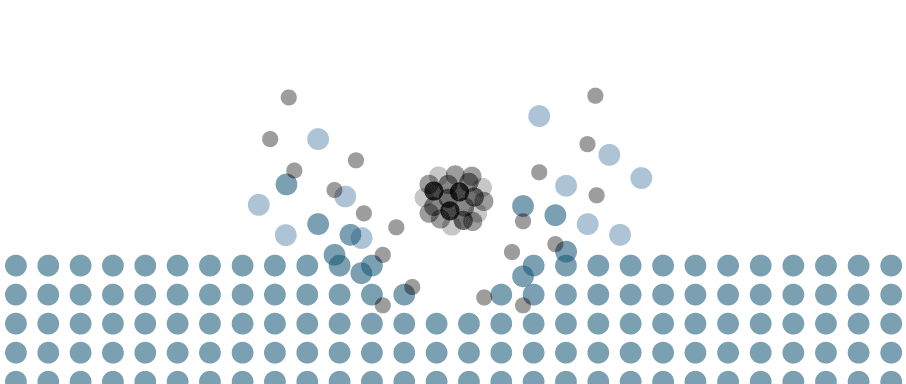
Gas cluster ion beams (GCIB) are high-energy beams of cluster ions, ideal for sputtering and analysing organic matter. GCIBs are an incredibly versatile ion source, as both the ion species and the beam properties can be varied, allowing the user to tune the beam to the needs of their experiment.
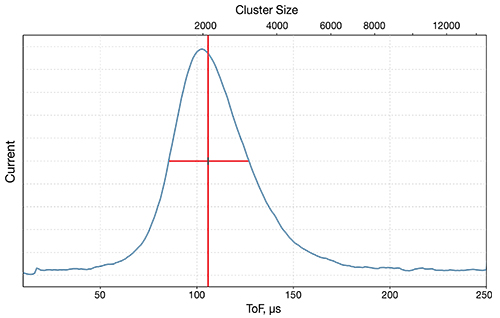
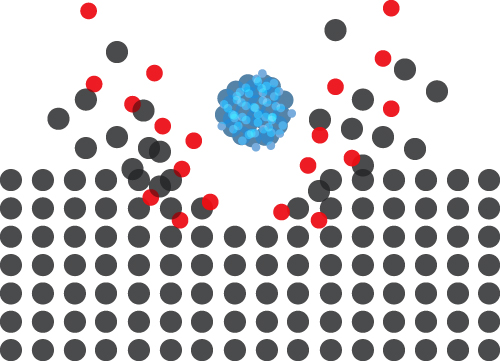
The source operates through the adiabatic expansion of gas in a vacuum, causing rapid cooling and cluster formation. The clusters are then ionised through electron bombardment and accelerated towards the target. The size of the cluster is a vital parameter, and users can adjust this over a wide range.
Organic Analysis
GCIBs are the ideal choice for sputtering organic matter. Etch rates of organic matter are orders of magnitude higher than for metals or semiconductors, making cluster beams such as the GCIB 10S an excellent tool for surface cleaning. The cluster distributes the ion’s energy across all constituent atoms/molecules, resulting in a very gentle sputtering effect and almost no damage to layers underneath—GCIBs perform much better than C60 on both fronts.
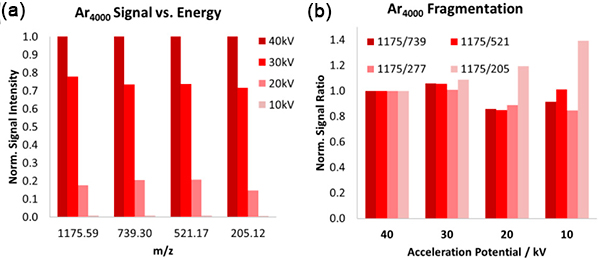
GCIBs must be operated at high energy to maximise their benefits for SIMS, as the secondary ion yield increases as a function of beam energy. We currently offer a 40kV variant, the GCIB 40, and a 70kV variant, the GCIB SM.
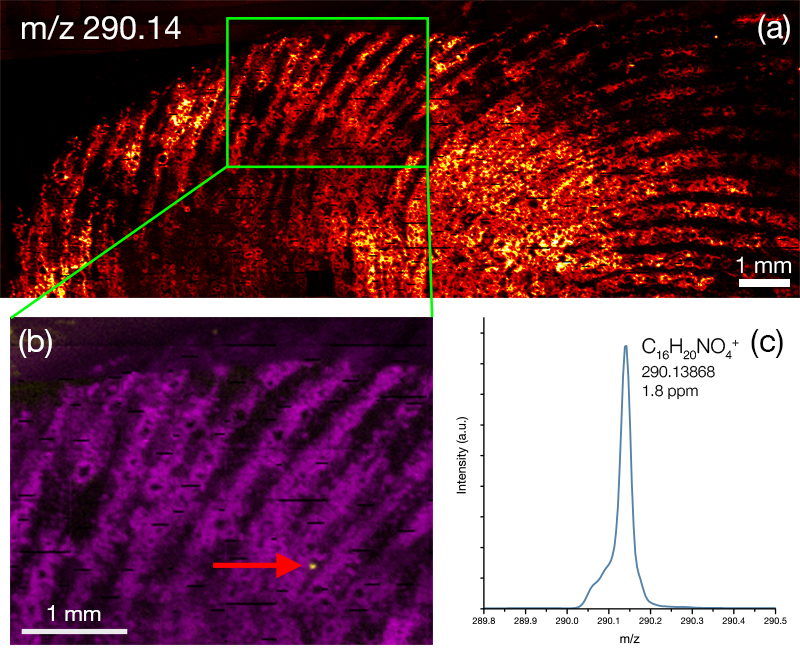
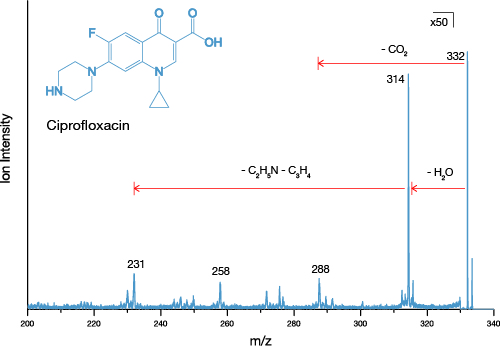
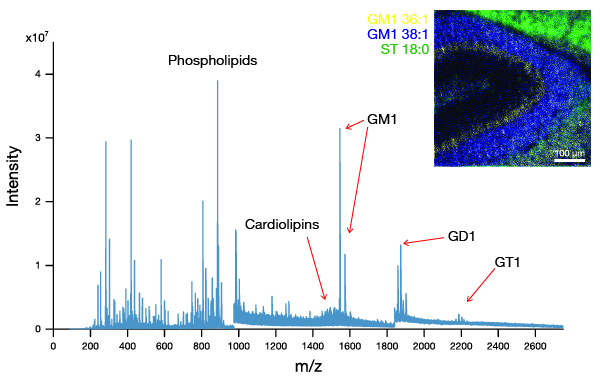
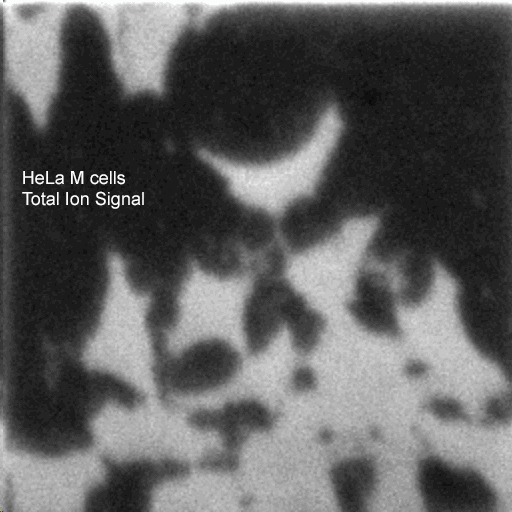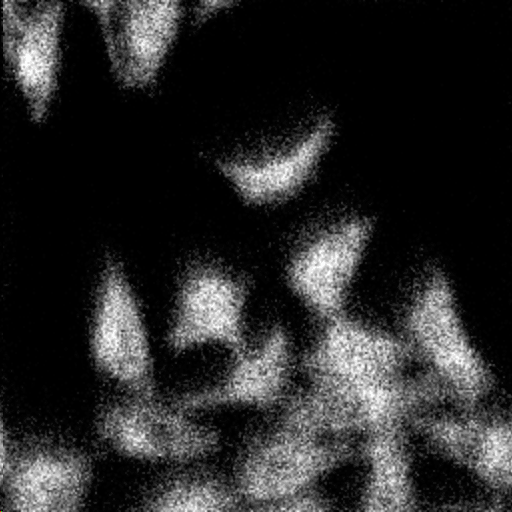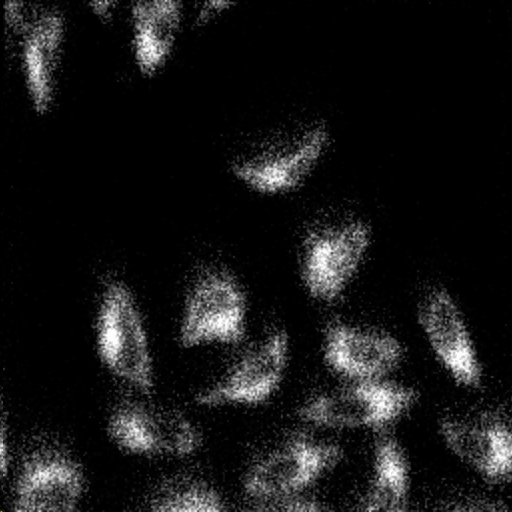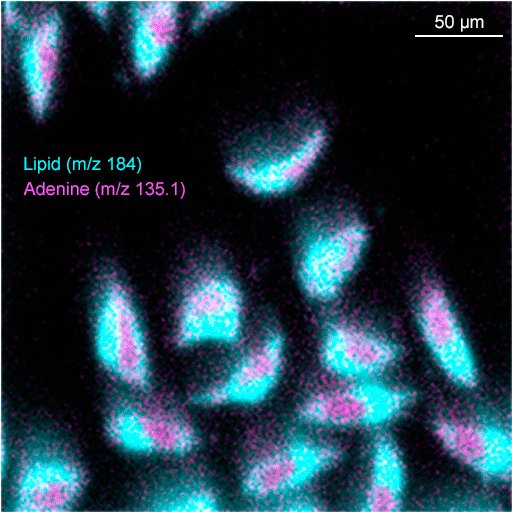
The J105 SIMS utilises the benefits of gas cluster beams for organic analysis. Combining the gentle sputter action of large cluster ions with increased secondary ion yield has extended the usable mass range to > m/z 2500.
Choice of gas
The versatility of GCIBs comes from having a choice of input gas. Argon is the most common as it is an inert gas that forms clusters easily, but Ar/CO2 mixtures and pure CO2 gas are also becoming standard for SIMS applications.
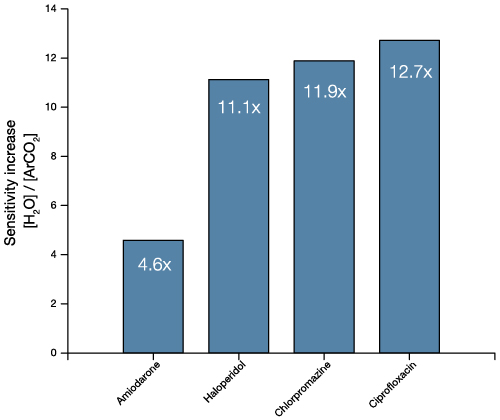
The stronger van der Waals forces between CO2 molecules result in much larger clusters than would be available for Ar – up to 20,000 in some cases. A wider range provides greater control of the all-important E/n value (energy per nucleon). Research has shown that optimising E/n results in an enhancement of the secondary ion signal. The presence of O– ions at the surface may also improve the ionisation probability – further enhancing ion yield.
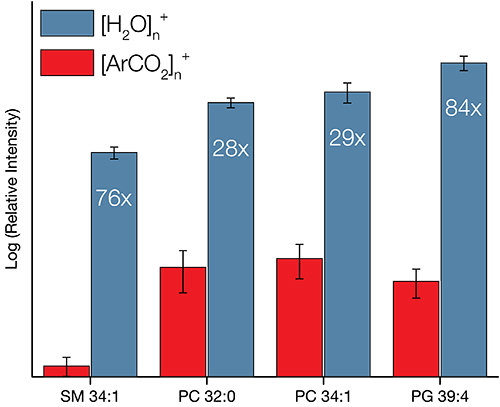
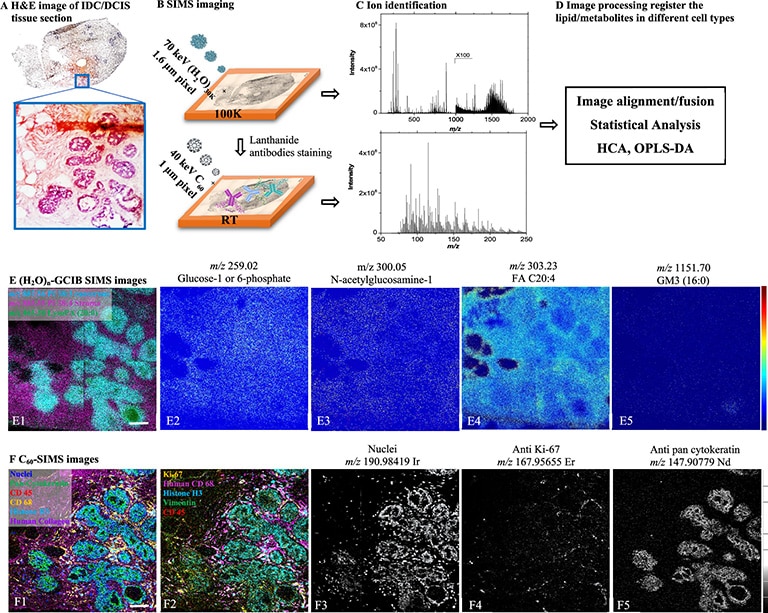
We have recently developed a GCIB source that runs on water vapour, which is currently available as an optional add-on for the J105 SIMS. Water molecules have even greater binding energy and can form enormous clusters of up to 60,000 molecules. Water clusters provide secondary ion yields up to 500 times greater than argon and are the best choice for state-of-the-art biological SIMS.
See our application note on choosing the best GCIB for your application for more detailed information.
Liquid Metal Ion Beams
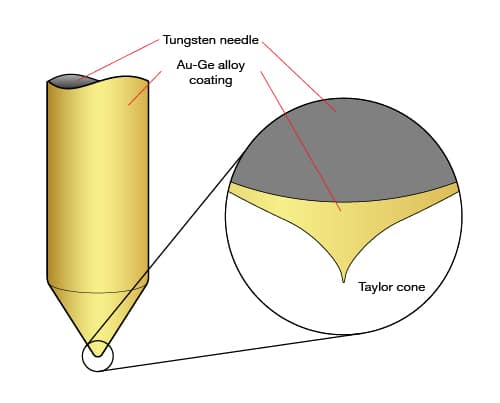
Liquid metal ion beams, also known as LMIS, or LMIG, are a well-established source technology. The source operates by a liquid metal reservoir feeding a blunt tungsten tip, from which a strong electric field extracts ions. Due to their elegant and reliable design, FIB systems have been using LMIS for decades. Ionoptika offers a 25 kV LMIG system in two variants; the IOG 25AU gold-cluster system and the IOG 25GA gallium system.
Liquid metal beams produce monatomic or small-cluster ion beams, such as Au+, Ga+, and Au3+. They feature high currents and small spot sizes (< 100 nm), making them ideal for high-resolution analysis applications.
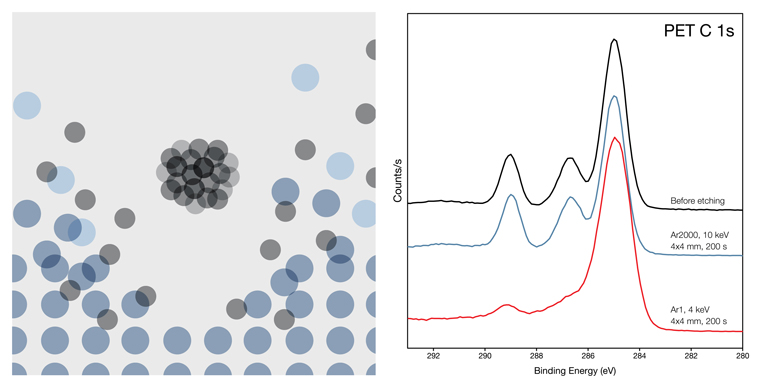
Small, high-energy ions can penetrate far beneath the surface before dissipating their energy. Known as channelling, this causes significant sub-surface damage, making depth profiling unreliable. It also results in considerable fragmentation, making LMIS more suited to analysing hard materials.
Plasma Ion Beams

Plasma ion sources are characterised by incredibly high brightness, making them ideal for high throughput applications. A single plasma source can run on various gases without changing parts, providing flexibility. Gases available for our plasma ion beams include hydrogen, helium, oxygen, nitrogen, argon, and xenon.
Plasma sources are monatomic and do not form clusters, resulting in lower energy distributions and smaller spot sizes. Combined with their high brightness, this leads to a very high current density beam.
Plasma beams are an excellent choice where the primary goal is high-volume etching or milling. For analysis purposes, plasma beams best suit harder materials such as metals, semiconductors, and inorganics.
FLIG – Floating Low Energy Ion Beam
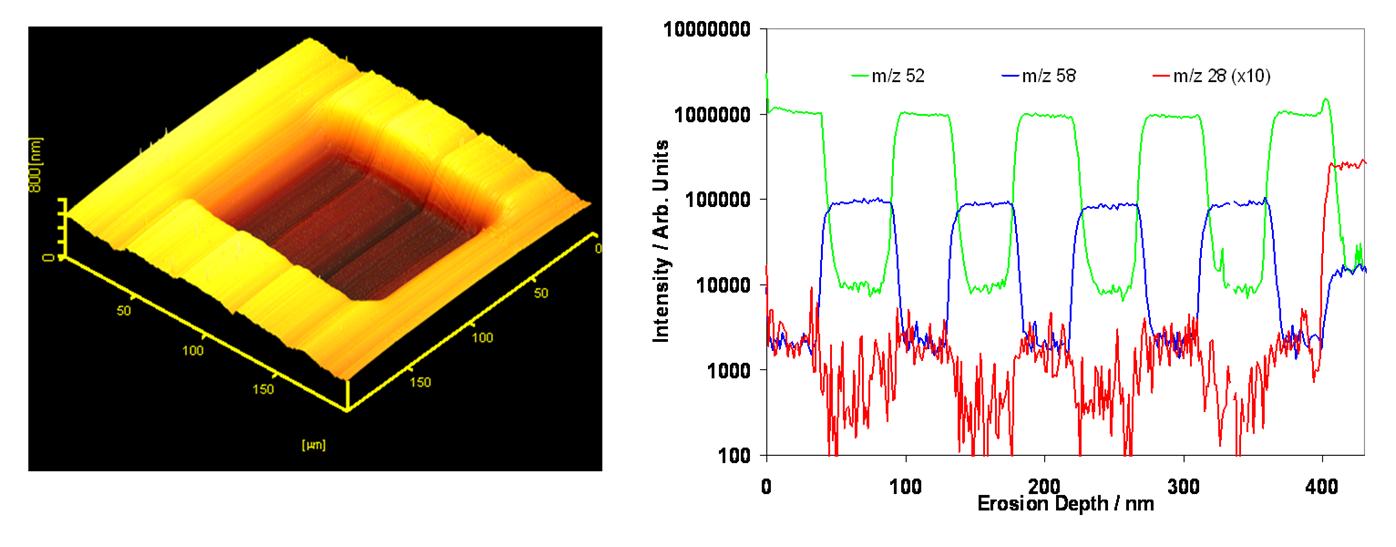
The FLIG 5 is a unique ion beam system based on a floating column design. The design enables ultra-low energy operation to 200 eV while still delivering a high current. Operating at such low impact energies significantly reduces the beam’s penetration depth, improving the depth resolution. Due to its high performance at ultra-low energies, the FLIG 5 has been the industry standard for shallow junction depth profiling for almost two decades.
Conclusion
The table below compares Ionoptika’s ion beam products under several categories discussed in this article (best viewed on desktop).
| ION BEAM | SPECIES | ENERGY RANGE | MIN SPOT SIZE | BEAM CURRENT | APPLICATION | BEST FOR | |
|---|---|---|---|---|---|---|---|
| C60 Ion Beams | |||||||
| C60-20S | C60+, C60++, C60+++ | 5 – 20 kV | 100 μm | 50 nA | SPUTTER | Organic, biological, inorganic, metals | |
| C60-20 | C60+, C60++, C60+++ | 5 –20 kV | 2 μm | 2 nA | ANALYTICAL | Organic, biological, inorganic, metals | |
| C60-40 | C60+, C60++, C60+++ | 10 – 40 kV | 300 nm | 1 nA | ANALYTICAL | Organic, biological, inorganic, metals | |
| Gas Cluster Ion Beams | |||||||
| GCIB 10S | Arn+, (CO2)n+, or (Ar/CO2)n+ | 1 – 10 kV | 250 μm | 60 nA | SPUTTER | Organic & biological, polymers | |
| GCIB 40 | Arn+, (CO2)n+, (Ar/CO2)n+, or (H2O)n+ | 5 – 40 kV | 3 μm | 200 pA | ANALYTICAL | Organic & biological, polymers | |
| GCIB 70/SM | Arn+, (CO2)n+, (Ar/CO2)n+, or (H2O)n+ | 20 – 70 kV | 1.5 μm | 300 pA | ANALYTICAL | Inorganic, organic & biological, polymers | |
| Liquid Metal Ion Beams | |||||||
| IOG 25AU | Au+, Au++, Au2+, Au3+, Au3++ | 5 – 25 kV | 100 nm | 10 nA | ANALYTICAL | Inorganics, metals, semiconductors | |
| IOG 25Ga | Ga+, 69Ga+ | 5 – 25 kV | 50 nm | 20 nA | ANALYTICAL | Inorganics, metals, semiconductors | |
| Plasma Ion Beams | |||||||
| IOG 30ECR | N2+, O2+, Ar+, & Xe+ | 5 – 30 kV | 500 nm | 500 nA | ANALYTICAL | Semiconductors, metals, inorganics | |
| IOG 30D | H2+, He+, N2+, O2+, & Ar+ | 5 – 30 kV | 500 nm | 500 nA | ANALYTICAL | Semiconductors, metals, inorganics | |
| FLIG 5 | H2+, He+, N2+, O2+, & Ar+ | 0.2 – 5 kV | 15 μm | 500 nA | ANALYTICAL | Semiconductors, depth profiling | |